Acetylcholinesterase (AChE) is a serine protease that plays an established role in cholinergic transmission by hydrolyzing the neurotransmitter acetylcholine, thereby terminating the synaptic transmission. However, the true challenge in the AChE research is the role of these enzymes in non-transmittive, non-synaptic phenomena. The notion that such unorthodox, non-classical phenomena must exist is clearly supported by several lines of evidence, such as the presence of AChE and other cholinergic components early before neurogenesis, and indeed in unfertilized and fertilized eggs and in the sperm of many species, and their presence throughout phylogenesis, including non-motile, monocellular organisms, fungi and plants and many anervous and ephemeral tissues.
During the course of our research, the enzyme AChE has become a central molecule of interest due to its ubiquitous functions and presence in various tissues and organisms.
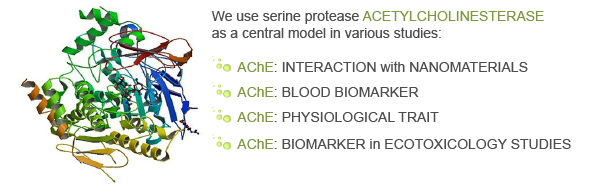
AChE molecular interaction with nanomaterials
We focus on the interaction of isolated AChE enzymes with nanomaterials. The idea is to study the adsorption and inhibition of AChE by different nanomaterials, which exhibit different absorption potential. In this way, AChE could be used as a biosensor for the ranking of NMs. At the moment, further work is needed to validate the proposed biosensor approach using different sources and isoforms of AChE. After acquisition of a larger set of data on the interaction of different NMs with different AChEs, it will be possible to connect the observed effects on AChEs to specific extrinsic properties of NMs.
Related content:
- Biological characterization of nanomaterials (Nanomaterial Characterization: An Introduction, 2016, book chapter)
- Toxic effects of carbon-based nanomaterials on acetylcholynesterase: An experimental and computational investigation (Toxicology Letters, 2013, 221: S245–S246)
- Effects of surface curvature and surface characteristics of carbon-based nanomaterials on the adsorption and activity of acetylcholinesterase (Carbon, 2013, 62: 222–232)
AChE as a blood biomarker
There are two different types of cholinesterase in human blood: acetylcholinesterase is found in the red blood cell membranes, and butyrylcholinesterase is found in blood plasma. We are interested in whether and how the blood activities of acetylcholinesterase and butyrylcholinesterase are influenced by different physiological conditions of human (for example exercise training). We also measure the AChE in mice of different ages.
Related content:
- Effect of maghemite nanoparticles on non-neuronal cholinergic system (2016, oral presentation); 5th Croatian Congress of Toxicology – CROTOX 2016, Poreč, Croatia
- Comparative study of serum protein binding to three different carbon-based nanomaterials (Carbon, 2015, 95: 560–572)
AChE as a physiological trait
Acetylcholinesterase is an enzyme with many known and unknown properties and functions. In particular, its role in the non-neuronal tissues remains as “an enigma of the cholinergic system”. This knowledge is particularly lacking in invertebrate physiology. We are interested how soluble and membrane AChE activity differ in the freshwater crustacean isopods Asellus aquaticus from the cave and surface environment. In the scope of this, we performed an extensive biomonitoring study of AChE activity in isopods collected from four different locations in three different seasons (spring, summer and autumn). In parallel to AChE, we also investigate the activity of glutathione S-transferase – a biomarker of detoxification activity, and energetic reserves.
Related content:
- Biomonitoring of water lice Asellus aquaticus from cave and surface freshwater environments (2015, conference poster); Cutting Edge, Faculty of Chemistry and Chemical Technology, Ljubljana, Slovenia
AChE as a biomarker in ecotoxicity studies
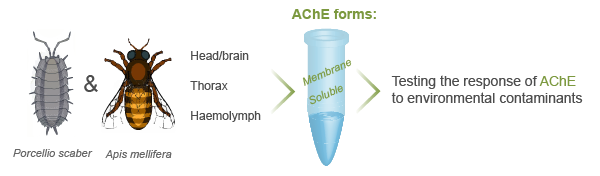
Acetylcholinesterase activity has been commonly proposed as a neurotoxicity biomarker for anticholinesterase chemicals such as organophosphorus and carbamate insecticides, as well as other classes of environmental contaminants like complex mixtures of metals, detergents, organic pollutants, and nanomaterials. We study the response of AChE from different organisms to chemical exposure. We particularly focus on the differential response of soluble and membrane AChE from different body regions (head, brain, thorax, and haemolymph).
Related content:
- Differential effects of organophosphate diazinon on membrane and soluble acetylcholinesterase in honeybee head and thorax (2016, conference poster); 7th Congress of Apidology – EurBee 2016, Cluj-Napoca, Romania
- Differential effects of ZnO and Ag nanoparticles, and diazinon on the activity of membrane and soluble form of acetylcholinesterase in honey bee head and thorax (2015, conference poster); 11th Conference on the Prevention of Honey Bee Colony Losses – COLOSS 2015, Lukovica, Slovenia
- Neurotoxic potential of ingested ZnO nanomaterials on bees (Chemosphere, 2015, 120: 547–554)
- Effects of selected metal oxide nanoparticles on Artemia salina larvae – evaluation of mortality and behavioural and biochemical responses (Environmental Monitoring and Assessment, 2014, 186: 4249-4259)
- Biochemical biomarkers in environmental studies – lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species (Environmental Science and Pollution Research, 2010, 17: 571-581)
- Toxicity of imidacloprid to the terrestrial isopod Porcellio scaber (Isopoda, Crustacea) (Chemosphere, 2008, 71: 1326–1334)
Methods
The AChE activity is assayed according to the colorimetric method developed by Ellman et al. (1961). This method is by far the most commonly applied method to measure the AChE activity. The nature of measured cholinesterases is checked using different types of inhibitors, such as eserine, neostigmin bromid, and other synthetic inhibitors.
Equipment and instrumentation
- Common biochemical equipment (e.g. homogenizers, centrifuges);
- Microplate reader (BioTek Cytation, Vermont, USA).